Acid-Base Titration Lab Report Chegg . Sign up to see more! Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. The analyte (titrand) is the solution with. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their. To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. Titration is an analytical quantitative technique used to determine the concentration of a solute; The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid. Use the mole ratio, which can be found using the balanced reaction. calculate how many moles of hci are used to neutralize the naoh.
from www.chegg.com
calculate how many moles of hci are used to neutralize the naoh. The analyte (titrand) is the solution with. Sign up to see more! Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their. To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. Use the mole ratio, which can be found using the balanced reaction. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid.
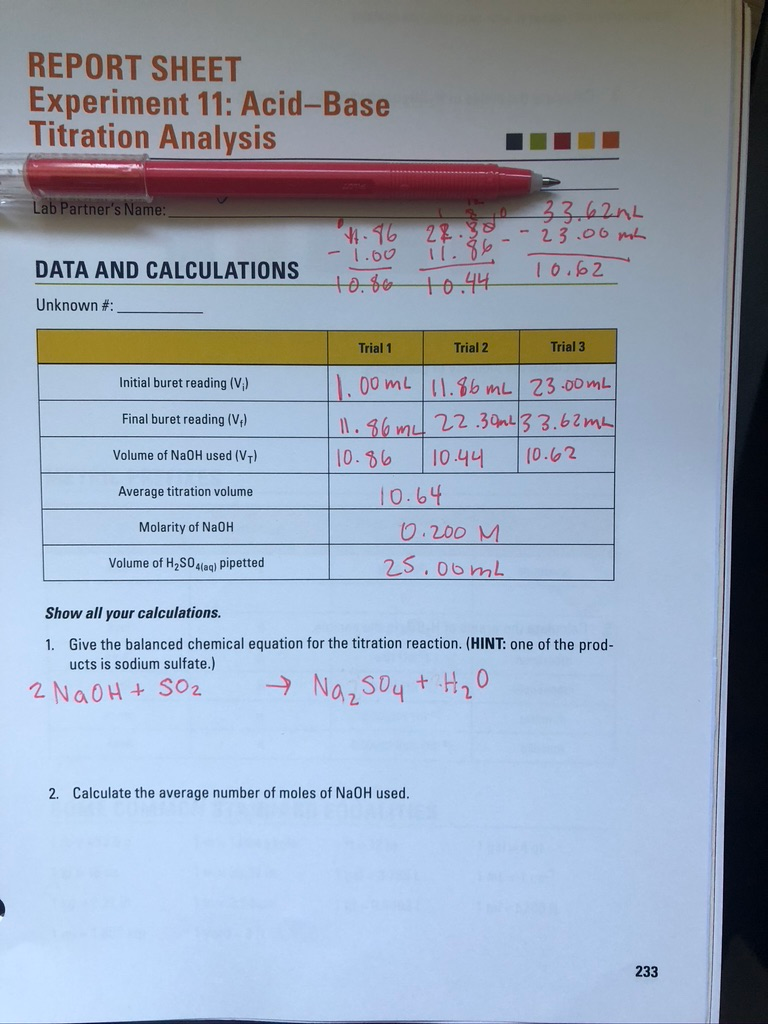
Solved REPORT SHEET Experiment 11 AcidBase Titration
Acid-Base Titration Lab Report Chegg To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. Sign up to see more! Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with. To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. Use the mole ratio, which can be found using the balanced reaction. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid. calculate how many moles of hci are used to neutralize the naoh.
From www.chegg.com
Solved Laboratory Experiment 4 Titrations of Acids and Acid-Base Titration Lab Report Chegg The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid. The analyte (titrand) is the solution with. Sign up to see more! Be able to determine the k a or k b from ph data associated with the titration of a weak. Acid-Base Titration Lab Report Chegg.
From www.coursehero.com
[Solved] Lab report on Acid base titration Course Hero Acid-Base Titration Lab Report Chegg Sign up to see more! The analyte (titrand) is the solution with. calculate how many moles of hci are used to neutralize the naoh. as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their. Be able to determine the k a or k b from ph data. Acid-Base Titration Lab Report Chegg.
From www.chegg.com
Solved Lab 12 Acid Base Titration Report Part 1 Acid-Base Titration Lab Report Chegg To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. The purpose of this. Acid-Base Titration Lab Report Chegg.
From www.chegg.com
REPORT SHEET LAB AcidBase Titration 12 A. Concent... Acid-Base Titration Lab Report Chegg as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid. The analyte (titrand) is the solution with. To determine the. Acid-Base Titration Lab Report Chegg.
From www.chegg.com
Dale Section Instructor REPORT SHEET LAB AcidBase Acid-Base Titration Lab Report Chegg calculate how many moles of hci are used to neutralize the naoh. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. The analyte (titrand) is the solution with. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with. Acid-Base Titration Lab Report Chegg.
From www.studocu.com
Experiment 7 Titrations Experiment 7 Titrations Required reading Acid-Base Titration Lab Report Chegg The analyte (titrand) is the solution with. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Titration is an analytical quantitative technique used to determine the concentration of a solute; The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid. Acid-Base Titration Lab Report Chegg.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Acid-Base Titration Lab Report Chegg as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their. To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. Sign up to see more! calculate how many moles of hci are used to neutralize the naoh. Titration. Acid-Base Titration Lab Report Chegg.
From mungfali.com
Acid Base Titration Lab Acid-Base Titration Lab Report Chegg calculate how many moles of hci are used to neutralize the naoh. Sign up to see more! Titration is an analytical quantitative technique used to determine the concentration of a solute; Use the mole ratio, which can be found using the balanced reaction. Be able to determine the k a or k b from ph data associated with the. Acid-Base Titration Lab Report Chegg.
From www.chegg.com
Solved Om WOUL REPORT SHEET LAB AcidBase Titration 20 A. Acid-Base Titration Lab Report Chegg Sign up to see more! The analyte (titrand) is the solution with. Use the mole ratio, which can be found using the balanced reaction. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Titration is an analytical quantitative technique used to determine the concentration of a. Acid-Base Titration Lab Report Chegg.
From www.hotzxgirl.com
Solved Report Sheet Acid Base Titration Lab Questions And Chegg Hot Acid-Base Titration Lab Report Chegg Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. Titration is an analytical quantitative technique used to determine the concentration of a solute; To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. calculate how many. Acid-Base Titration Lab Report Chegg.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Acid-Base Titration Lab Report Chegg Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium. Acid-Base Titration Lab Report Chegg.
From www.chegg.com
Solved PART B Acid Base Titration Lab Report I Acid-Base Titration Lab Report Chegg calculate how many moles of hci are used to neutralize the naoh. Use the mole ratio, which can be found using the balanced reaction. The analyte (titrand) is the solution with. To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. Sign up to see more! The purpose of this. Acid-Base Titration Lab Report Chegg.
From exoqbgfse.blob.core.windows.net
Sample Lab Report On Acid Base Titration at Michelle Hamilton blog Acid-Base Titration Lab Report Chegg Sign up to see more! calculate how many moles of hci are used to neutralize the naoh. To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. The analyte (titrand) is the solution with. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid. Acid-Base Titration Lab Report Chegg.
From webapi.bu.edu
Discussion acid base titration lab report. Acid Base Titration Lab Acid-Base Titration Lab Report Chegg The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; as seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively. Acid-Base Titration Lab Report Chegg.
From www.chegg.com
Solved volumetric analysis titrations with acids and bases Acid-Base Titration Lab Report Chegg The analyte (titrand) is the solution with. Use the mole ratio, which can be found using the balanced reaction. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid. Sign up to see more! To determine the volume of naoh used in. Acid-Base Titration Lab Report Chegg.
From exoqbgfse.blob.core.windows.net
Sample Lab Report On Acid Base Titration at Michelle Hamilton blog Acid-Base Titration Lab Report Chegg Be able to determine the k a or k b from ph data associated with the titration of a weak acid or base. calculate how many moles of hci are used to neutralize the naoh. The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic. Acid-Base Titration Lab Report Chegg.
From www.chegg.com
Solved Titration for Acetic Acid in VinegarLab Report Acid-Base Titration Lab Report Chegg Use the mole ratio, which can be found using the balanced reaction. To determine the volume of naoh used in each trial, subtract the initial naoh level in the buret from. Sign up to see more! Titration is an analytical quantitative technique used to determine the concentration of a solute; calculate how many moles of hci are used to. Acid-Base Titration Lab Report Chegg.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Acid-Base Titration Lab Report Chegg The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of. Acid-Base Titration Lab Report Chegg.